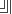1- Sublevel (d) has 5 orbitals.
2- Sublevel (f) has 7 orbitals.
3- Orbitals of the same sub level are equal
in energy and shape.
Ex: px
= py = pz
4- No of orbitals in each E. level = square
order of E. level = n2.
1-
Spin
Q. No (ms):
·
Detects
the direction in which the electron spins around its axis during its rotation
around the nucleus.
·
 Each orbital can be saturated by 2 electrons, one
electron spins around its axis clockwise while the other electron spins anti –
clockwise in order to from 2 opposite magnetic fields to decrease the force of
repulsion between them which keep the atom stable.
Each orbital can be saturated by 2 electrons, one
electron spins around its axis clockwise while the other electron spins anti –
clockwise in order to from 2 opposite magnetic fields to decrease the force of
repulsion between them which keep the atom stable.
·
It
has only two possible values + 1/2 – 1/2
Give reason: Each orbital carries 2
electrons although they are negatively charged.
Summary of the relationship between the principle E.L,
sub levels orbitals and no of electrons:
1- No of energy sublevels = order of
principle level (n).
2- No of orbitals within a principle level
= square the no of the level (n2).
3- No of electrons occupying a given E.
level = two times the square order of this
level (2n2).